α-Pinene: A Deep Dive into Its Journey, Uses, and Challenges
Historical Development
Long before α-pinene appeared on chemical inventory spreadsheets or regulatory lists, people encountered it in pine forests. Walk into a conifer grove anywhere in the world, you’ll catch its sharp, refreshing scent. Shipbuilders figured out centuries ago that pine resin, heavy with α-pinene, worked as a waterproofer and adhesive. European apothecaries listed pine oil as a medicine during the Renaissance. The distillation of turpentine for lamp fuel and solvents established a global trade by the 1700s, and chemists learned to isolate and name its main components, with α-pinene standing out as one of the most abundant. Over time, industries started valuing this monoterpene for more than its aroma, leading to refinement of its extraction and use in everything from paint thinners to medications.
Product Overview
α-Pinene is a bicyclic monoterpene that dominates the volatile world of pine, fir, spruce, and even some herbs like rosemary and basil. It’s a clear, colorless liquid in pure form, flashing a scent that’s immediately familiar to anyone who’s walked near pine trees. Commercial α-pinene usually comes from turpentine, which gets distilled from pine resins collected in large forested regions, especially in the southeastern United States, China, and Brazil. Producers and end-users look for consistency in odor, low levels of contamination, and the right enantiomer for their specific process needs, since α-pinene comes in two mirror-image forms: the (−)-α-pinene and (+)-α-pinene.
Physical & Chemical Properties
This compound has a boiling point near 156°C and freezes at −62°C. With a density around 0.86 g/cm³ and low solubility in water, α-pinene floats, evaporates readily, and tends to separate unless emulsified. Chemically, it’s pretty reactive thanks to the strained four-membered ring in its structure. Exposure to air, light, heat, and certain acids can convert it to a pile of different terpenes, including camphene, limonene, or pinene oxide. Flammability is a concern, as with many essential oil components—store this liquid away from sparks and open flames.
Technical Specifications & Labeling
Quality standards for α-pinene often follow protocols set by organizations such as ASTM and ISO. Details matter: percent purity (usually above 95%), ratio of isomers, presence of residual solvents, and other terpenes all get measured. Industry labels specify “(−)-α-pinene” or “(+)-α-pinene”, reflecting their optical activity, and containers require hazard statements about flammability and inhalation exposure. Tracking batch numbers helps manage recalls and audits. Material Safety Data Sheets (MSDS) tell operators how to handle spills or accidental contact. For those making flavors, fragrances, or pharmaceuticals, regulatory approvals from agencies such as the FDA or European Chemicals Agency are essential, since the wrong impurity or mislabeling could cause major problems downstream.
Preparation Method
Distillation stands as the mainstay for producing α-pinene. Producers tap pine trees for resin, collect gum turpentine, and distill it, taking care to avoid overheating, which triggers unwanted side reactions. Fractional distillation or vacuum distillation can increase purity. For laboratory work or specialty applications, chemists sometimes separate isomers using methods like chiral chromatography. Advances in microbial biotechnology have sparked some interest in engineering bacteria or yeasts to build α-pinene from renewable feedstocks like glucose, but these remain niche and tend to cost more than forest-derived production. The preference for large-scale, cost-effective approaches keeps natural distillation at the center of the supply chain.
Chemical Reactions & Modifications
α-Pinene opens the door to a host of chemical transformations. Acidic conditions can isomerize it to camphene, which finds plenty of use in perfume and insecticide synthesis. Ozonolysis or reactions with other oxidants can yield pinene oxide, verbenone, or a set of smaller aldehydes and ketones prized as flavoring agents. Hydrogenation reduces α-pinene to pinane, useful in making high-octane fuels or solvents. Labs and factories turn to this chemistry thanks to the availability and reactivity of α-pinene, essentially making it a renewable “starter block” for specialty chemicals. Every year, companies patent new derivatives, each taking advantage of this basic monoterpene’s highly strained ring system and easy modifications.
Synonyms & Product Names
On shipping manifests or scientific catalogs, α-pinene might appear under several names: 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene, (−)-α-pinene, (+)-α-pinene, or simply Pin-2-ene. Turpentine oil and Spirit of Turpentine often denote crude mixtures heavy in α-pinene. Brands bottle it under proprietary names when selling to the fragrance, pharmaceutical, or agricultural sector, usually accompanied by a technical identifier, a batch code, and certification about the absence of banned substances.
Safety & Operational Standards
Handling α-pinene means thinking about fire risk, vapor inhalation, and skin irritation. Factories emphasize sealed vessels, explosion-proof extraction systems, and proper ventilation. Workers wear gloves, respirators, and eye protection during distillation, transfer, and packaging. Hazard labelling must comply with widely recognized guidelines, including pictograms and clear written warnings. Spills get treated as both environmental and fire hazards: sand or absorbent controls small spills, but larger releases near drains can harm aquatic life. Fire suppression systems in facilities housing α-pinene double as insurance against legal, human, and financial disaster.
Application Area
Few natural molecules show up in as many industries as α-pinene. Perfumers rely on its fresh aroma to anchor pine-inspired scents or mimic forest bouquets. Food processors blend it into flavors for baked goods, candies, and vapes, again chasing that authentic “pine” note. Pharmaceutical firms convert it into camphor and other active ingredients for topical gels, cough suppressants, and decongestants. Cleaning product formulators fold α-pinene into degreasers and disinfectants—its solvent power beats out many harsh synthetics, and consumers trust the “natural” angle. Crop protection companies use α-pinene to build biodegradable insecticides and herbicides, which sometimes face easier regulatory hurdles than purely synthetic options. You’ll spot traces of it in adhesives, rubber additives, and even printing inks, where volatility and clean burnout count as major assets.
Research & Development
Scientists keep exploring new uses and production tweaks for α-pinene. Green chemistry projects target enzymes and bacteria as future bio-factories, hoping to outdo the slow, environmentally destructive harvest of wild pine. Drug researchers examine α-pinene for reported anti-inflammatory, bronchodilator, and antimicrobial effects, looking to validate or debunk claims that go back to folk medicine. Meteorologists and environmental chemists track what happens when α-pinene floats up from forests, reacts with atmospheric compounds, and shapes cloud formation or air quality. Polymer scientists and materials engineers see α-pinene as a building block for “greener” plastics and coatings, spurred by both consumer demand and regulatory pressure.
Toxicity Research
While α-pinene appears in natural settings all the time, concentrated exposures raise questions. Animal studies show it can irritate eyes, skin, and mucous membranes. High inhalation exposures lead to headaches, nausea, or drowsiness in humans. Chronic or massive overexposure remains poorly studied, partly because real-world exposures rarely reach such levels outside factory environments. Regulatory agencies including the U.S. EPA and the European Chemicals Agency keep α-pinene within the less-risky class of industrial solvents and aromas, but they watch for new toxicity data. Direct environmental toxicity appears low—most pinene evaporates or biodegrades, posing little risk of groundwater contamination or food chain buildup. Still, accidental spills in water can suffocate fish or aquatic organisms if concentrations climb high enough.
Future Prospects
Few natural-product chemicals have as much unlocked potential as α-pinene. Increasing global demand for flavors and “green” chemicals looks set to expand output, and new microbial and plant-based synthesis methods could shake up who controls supply. Consumer preferences for natural aromas, regulatory pressure against petroleum-derived solvents, and the constant need for novel bioactives keep industrial interest steady. The main challenges lie in ensuring sustainable sourcing, reducing waste, and developing processes that require less water, energy, or hazardous chemicals. As biomedical research continues, hopes rise for strong evidence to back up long-standing medicinal claims about α-pinene. The question isn’t whether industry will keep using α-pinene, but how producers will balance expanding demand with safety, sustainability, and stricter global rules. Looking at the ingenuity shown so far, odds favor ongoing adaptation and surprise breakthroughs in how this forest-scented molecule shapes technology and health.
Nature’s Aromatic Workhorse
Pine trees do more than freshen up Christmas mornings; they push out a compound called α-Pinene. This molecule, part of what gives forests that sharp, crisp smell, doesn’t just sit in the woods waiting for hikers. Commercial producers pull α-Pinene out of turpentine oil, bringing the outdoors right into labs and factories.
Fresh Scents and Clean Homes
I remember the smell of freshly cleaned floors at my grandmother’s house. The piney bite in the air didn’t come from any fancy perfume—it came from cleaning products full of α-Pinene. Companies rely on it to give cleaners that unmistakable scent of cleanliness and nature. Research shows that people associate these pine notes with health and hygiene, so brands keep bottling the smell year after year.
Medicine’s Subtle Helper
Herbal medicine often circles back to old wisdom. Folk remedies used pine for coughs and congestion, and scientists found some of that is due to α-Pinene’s impact on airway passages. Studies highlighted its anti-inflammatory abilities, especially for folks struggling with stuffy noses or seasonal bugs. On top of that, there’s evidence that it works as a mild bronchodilator, opening up the breathing tubes just a bit wider. While not a cure-all, it plays a sturdy supporting role in cough drops, essential oils, and even some topical creams for soreness.
Flavors, Fragrances, and Food
I once tried a craft gin that tasted like a forest in a bottle. That sharp note didn’t just come from juniper — it often comes from a splash of α-Pinene. Food scientists lean on it to give beverages and candies a piney, herbal twist. On the fragrance side, perfumers say it’s hard to duplicate that earthy, lively hit found in pine needles, so they stick with nature’s own molecule to give perfumes and air fresheners that authentic snap.
Green Chemistry: Building Blocks for Everyday Stuff
α-Pinene doesn’t stop at taste and smell. Chemical engineers use it as a stepping stone, turning it into important industrial goods. For example, it helps form camphor, which finds its way into medicinal ointments and some plastics. Down the line, manufacturers turn it into synthetic pine oil, linalool, and other ingredients found in everything from bug repellents to printable inks. As the world looks for plant-based alternatives, α-Pinene offers an option that reflects a move away from fossil fuels.
Sustainable Choices and Challenges
Using tree-derived products has an impact. Big producers must look after forests, or risk stripping away the very supply that keeps the chain going. I’ve spoken with foresters who say that careful, slow harvesting makes for a healthier landscape and supports rural jobs. Keeping α-Pinene production tied to sustainable practices protects both the market and the wild places people value, creating a reason to look after those green spaces just as much as the finished goods on store shelves.
Looking Ahead
Seeing α-Pinene’s journey from old pine stands to modern medicine cabinets makes it clear that useful, effective solutions can come from nature. With smart science, good stewardship, and a respect for the source, this once humble chemical keeps proving its value across industries and daily life alike.
How α-Pinene Shows Up in Daily Life
Stroll through a pine forest and the fresh, sharp scent you pick up in the air often comes from α-Pinene. This compound gives pine needles, rosemary, and even some herbs their recognizable aroma. Grown folks with a backyard or garden have likely smelled it too, snipping rosemary or crushing basil for a meal. Manufacturers pull α-Pinene from plants by steam distillation and add it into flavors, fragrances, and even certain health products. Marketing sometimes spins it as a “natural” option for everything from cough syrups to food flavoring.
What We Know From the Science
Research backs up some traditional uses of α-Pinene. Studies show it can help open airways and fight certain germs. Inhaling it in the forest is common for a reason—Japanese “forest bathing” and similar practices claim health benefits, thanks in part to volatile compounds like this one. Food scientists, meanwhile, have long listed α-Pinene as a flavoring, with the U.S. Food and Drug Administration including it in the category of substances generally recognized as safe (GRAS) when used within specific limits.
That GRAS status rests on toxicology studies. Animal tests using reasonably low doses, alongside many years of humans eating trace amounts from common herbs, suggest α-Pinene isn’t dangerous in small quantities. In one study, rats exposed to high doses for several weeks showed some minor changes to liver and kidney tissue, but those effects happened at levels much higher than a typical person would encounter from food or air.
Unusual Situations and Real Risks
Stories of people ending up with problems after eating foods rich in α-Pinene are rare. What does pop up now and then are calls to poison control for kids or adults who tried to swallow neat pine essential oil. Direct consumption in larger amounts can trigger nausea, vomiting, or, in the rarest cases, problems with the nervous system. I’ve helped my own kids learn to read essential oil labels carefully after hearing from friends who were tempted by product trends on social media.
Contact allergies also crop up, usually when people use pine oil in skin products or cleaning agents. Rashes or mild swelling might follow but eating herbs in regular dishes rarely triggers this kind of reaction. People with specific allergies—those who know they react badly to pine or turpentine—should of course avoid it altogether.
What Makes Safe Use Possible
Safety standards make a real difference. Food companies use α-Pinene in such small amounts that aroma or flavor comes through but toxicity remains off the table. Flavors and fragrances undergo review in many countries, with the European Food Safety Authority also allowing use as a flavoring, again within controlled limits. The FDA advises against consuming pine needle oil directly and asks that companies stick to strict rules for purity and dosage. Problems arise mostly from do-it-yourself remedies that skip these checks.
A Sensible Approach
Real peace of mind comes from knowing where the α-Pinene in a product comes from, and how much sits inside. Most kitchen herbs, teas, or flavorings keep the dose far below any danger point. Health authorities stick to conservative limits to protect kids and folks with conditions like asthma or allergies. Anyone looking to take new supplements or ingest concentrated oils can talk things through with a pharmacist or medical provider.
The world offers many aromatic compounds. α-Pinene, in small doses as part of whole foods or in regulated products, earns its track record for safety. Staying smart means sticking to intended uses, keeping essential oils out of reach of curious kids, and reading labels with care.
Where α-Pinene Comes From
Ask anyone who has crushed a pine needle or snapped a fresh rosemary sprig, and they'll know the sharp, crisp scent that greets the nose. That same scent carries a vital clue: it comes from α-pinene. This compound isn’t some mystery chemical cooked up in a lab. It exists throughout nature, especially in coniferous trees like pines, firs, and spruces. Forestry workers and hikers get a literal breath of α-pinene with every walk through a dense forest.
Beyond pine forests, α-pinene pops up in herbs such as rosemary, sage, and basil. Its presence doesn’t stop at herbs or trees. Some citrus peels also carry this compound, proof that nature likes to remix its chemistry in more ways than one.
The Role of Trees and Nature
I’ve spent years outdoors, sometimes gathering wood, sometimes just out for a long walk. What stands out is how many trees, especially the sticky pine varieties, ooze resin that smells both spicy and fresh. This sticky resin is where α-pinene concentrates, serving a clear purpose for the plant. Resin protects trees from insects and pathogens. It acts as a natural defense system, hardening on wounds and sealing off entry points.
The bulk of commercial α-pinene comes from the processing of turpentine. Turpentine starts as the resin tapped from living pine trees or as a byproduct of the pulping industry. Those in forestry understand the routine: tap the tree, collect the resin, distill it, and pull out the α-pinene. Every time I’ve handled these resins, the distinctive sharp, green odor is impossible to miss. This isn’t some distant, industrial process; it’s built on the same interactions humans have had with trees for generations.
Why α-Pinene Matters in Everyday Life
Almost everyone has encountered α-pinene, often without realizing it. It shows up in everything from household cleaners to perfumes and even some medicines. Those cleaning products with a “pine-fresh” scent almost always rely on this compound. I remember working in a carpentry shop, noticing the strong aroma coming from wood shavings and sawdust. That wasn’t just the smell of hard work but the natural punch from compounds like α-pinene.
More interestingly, recent research highlights how α-pinene might offer benefits for human health. Some studies suggest it can act as an anti-inflammatory, and there’s talk about its value in traditional and modern remedies. Breathing forest air rich in α-pinene produces what’s known as “forest bathing” or “shinrin-yoku” in Japan, a practice now supported by growing scientific evidence on mood and immune benefits. That soothing, revitalizing effect people get isn’t just sentimental; there’s real chemistry behind it.
How to Support Responsible Sourcing
Over-harvesting trees for resin can stress forests. That’s a real concern, as anyone who’s watched a clear-cut forest knows. Sustainable forestry practices, such as selective tapping and responsible replanting, keep the balance. Certification standards, including those from the Forest Stewardship Council (FSC), help ensure α-pinene extraction doesn’t come at the cost of ecosystem health.
Synthetic production of α-pinene also exists, but natural sources remain valuable due to their lower environmental impact if managed smartly. Pressing industries to pay attention to how and where they source their turpentine resin matters. Consumers play a part by supporting products with strong certification and clear sourcing claims.
Why α-Pinene Deserves Respectful Storage
α-Pinene is more than a pine-scented chemical; its presence in daily life stretches from flavorings to cleaners. What surprises many is how tricky it can get once you’re in charge of storing it. α-Pinene is a volatile organic liquid, which means it loves to evaporate and doesn’t mind catching fire. That smell you notice from a leaky bottle quickly becomes a safety risk in the wrong conditions. Over the years, I learned what happens when someone ignores the basics: leaky lids, odd smells in the storeroom, or a flash of flame from static—it all points to taking shortcuts.
Understanding Its Personality
α-Pinene boils at just above 150°C, but even at room temperature, you’ll see it disappear into thin air if a container isn’t sealed right. Its vapor travels, sometimes across the room, mixing with air and clinging to surfaces. Once mixed with oxygen, the risk of forming explosive mixtures rises. I once worked with a batch stored in old glass jars; two weeks later, the concentration in the storeroom triggered alarms. Unsealed or poorly closed containers will guarantee headaches—literal and regulatory.
Best Practices for Storage—Drawn From Experience
People often ask whether stainless steel or glass is best. I’ve seen both succeed, but I always reach for tightly sealed metal drums or dark glass bottles for small amounts. Exposure to sunlight doesn’t just fade the liquid; it changes α-Pinene’s chemistry, making it less useful and sometimes even hazardous after a few months. If left open, oxygen does its work, turning it sticky and resinous, which ruins valuable product and fouls storage shelves.
Temperature ControlKeeping α-Pinene cool slows down its urge to evaporate, so room temperature or below works well. Never place it near heaters, electrical equipment, or open flames. Warm environments encourage pressure buildup inside containers. I recall a summer where a storage closet hit 35°C. One metal jug buckled, leaking its contents and filling the air with a strong pine odor nobody enjoyed.
The Importance of Ventilation and Fire SafetyVentilation matters as much as temperature. Without fresh air, vapors stick around, waiting for a stray spark. A proper chemical storage cabinet with built-in venting has bailed out more than a few colleagues. Fire extinguishers suited for flammable liquids (foam, dry powder, or CO₂) belong nearby, not across the building. This isn’t just legal red tape—quick access can stop a small mishap from turning into a disaster.
Labeling and Training—Respect for RulesClear labels help everyone, not just the safety team. Mark containers with the chemical’s name, hazard symbols, and a date, using waterproof ink. Mixing up pine oil with paint thinner leads to confusion or dangerous combinations. Beginners need training—understanding what goes wrong in the field often keeps people out of the emergency room.
Common Sense, Not Just Rules
Every storeroom or laboratory brings its quirks. Still, storing α-Pinene in small, sealed, labeled containers, away from light and heat, cuts risks to almost nothing. Nobody wants to leave work smelling like a pine forest because someone skipped a step. Prevention beats clean-up, every time.
Understanding α-Pinene
Pine trees fill forests with their fresh, sharp scent. That signature aroma comes from α-pinene, a natural compound present in many coniferous trees, rosemary, basil, and some citrus peels. People have leaned on these scents for centuries, whether burning pine branches in rituals or brewing herbal infusions. Science peers deeper, turning to α-pinene’s chemistry for answers about its health effects.
A Breath of Fresh Air for the Lungs
Stepping into a pine forest, breathing feels easier. Research backs up this sense, as α-pinene may reduce airway inflammation. Asthma or chronic bronchitis limits daily life, but animal models show α-pinene could help with less severe symptoms and fewer flare-ups. It serves as a bronchodilator, letting the lungs draw in oxygen more freely. Clinical trials remain early, yet the story fits with the comfort many feel while hiking through a wooded trail.
Defending Against Microbes
Nature arms its trees with α-pinene to fend off bacteria and fungus. Human bodies aren’t much different and benefit from similar support. Lab studies find that α-pinene blocks the growth of several bacteria, including Staphylococcus aureus. While this cannot replace antibiotics, there’s promise in using natural compounds as part of broader solutions—think essential oils in wound care or as part of a cleaning routine in homes.
Soothing Minds Under Stress
Modern life brings more stress than restorative time in the forest. Research hints α-pinene may have calming effects on the nervous system. Some studies show it can support mental clarity and lift mood, possibly by influencing neurotransmitter levels in the brain. Traditional uses of pine needle tea fit here, as people often turn to such remedies to care for nerves and promote relaxation. No single compound erases anxiety, but incorporating α-pinene-rich environments or products may support a more peaceful daily routine.
Anti-Inflammatory Power
Inflammation lies behind many chronic health conditions, including arthritis and some heart problems. α-Pinene demonstrates strong antioxidant and anti-inflammatory properties in laboratory tests. Researchers find that it can block inflammatory pathways, cutting down the chemical signals that trigger swelling and pain. Herbalists have used pine extracts for joint discomfort for generations, and science is beginning to catch up by exploring α-pinene as a natural support.
Safeguarding the Brain
Memory sometimes falters as people age, raising questions about natural support for brain health. α-Pinene could help defend neurons against damage. Early research links the compound with better memory scores and less brain cell stress in animal models. Scientists believe it may shield nerve cells from the inflammation and oxidative stress tied to neurodegenerative diseases. While more studies in humans are needed, these early findings give hope for preserving sharpness and slowing the march of memory loss.
Building Smarter Solutions
No single forest extract serves as a cure-all. Embracing α-pinene fits with a broader move to recognize nature’s toolkit and blend it with medical advances. Support for lung and brain health, mood resilience, and infection control all gains ground with a focus on both scientific evidence and tradition. As always, natural remedies require respect—attention to proper dosing, careful sourcing, and more clinical trials will help bring balance between old wisdom and new knowledge.
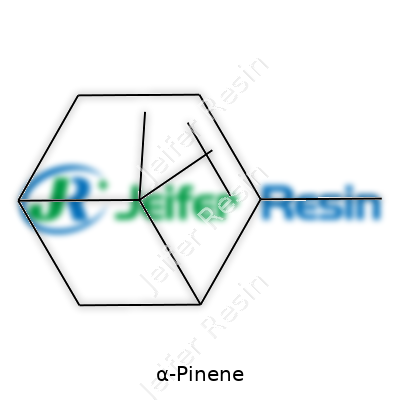
| Names | |
| Preferred IUPAC name | (1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene |
| Other names |
Alpha-Pinene
α-Pinen 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene Pinene Pinen Pinol A-Pinene |
| Pronunciation | /ˈæl.fə ˈpaɪniːn/ |
| Identifiers | |
| CAS Number | 80-56-8 |
| Beilstein Reference | 1905031 |
| ChEBI | CHEBI:17514 |
| ChEMBL | CHEMBL49020 |
| ChemSpider | 9696 |
| DrugBank | DB14074 |
| ECHA InfoCard | ECHA InfoCard: 100.003.464 |
| EC Number | EC 201-291-9 |
| Gmelin Reference | 591 |
| KEGG | C06425 |
| MeSH | D010866 |
| PubChem CID | 6654 |
| RTECS number | SD6469500 |
| UNII | 9O2L8MZH3E |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C10H16 |
| Molar mass | 136.24 g/mol |
| Appearance | Colorless to pale yellow liquid with a distinctive pine-like odor. |
| Odor | pine-like |
| Density | 0.858 g/mL at 25 °C |
| Solubility in water | 0.025 g/100 mL |
| log P | 2.99 |
| Vapor pressure | 4 mmHg (25°C) |
| Acidity (pKa) | 19.18 |
| Basicity (pKb) | 14.10 |
| Magnetic susceptibility (χ) | -67.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.464–1.467 |
| Viscosity | 3.73 mPa·s (at 25 °C) |
| Dipole moment | 2.115 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 347.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -229.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4786 kJ/mol |
| Pharmacology | |
| ATC code | D02AE04 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H304, H315, H317, H410 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P272, P273, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P331, P332+P313, P333+P313, P337+P313, P362+P364, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-2-Ν |
| Flash point | 33 °C |
| Autoignition temperature | 233 °C |
| Explosive limits | 0.8–6% |
| Lethal dose or concentration | LD50 oral rat 3.7 g/kg |
| LD50 (median dose) | LD50 (median dose) of α-Pinene: 3,700 mg/kg (rat, oral) |
| NIOSH | NIOSH: UR7550000 |
| PEL (Permissible) | 100 ppm (TWA) |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 900 ppm |
| Related compounds | |
| Related compounds |
Camphene
Sabinene Limonene Myrcene Beta-Pinene Terpinolene Pinene oxide |



